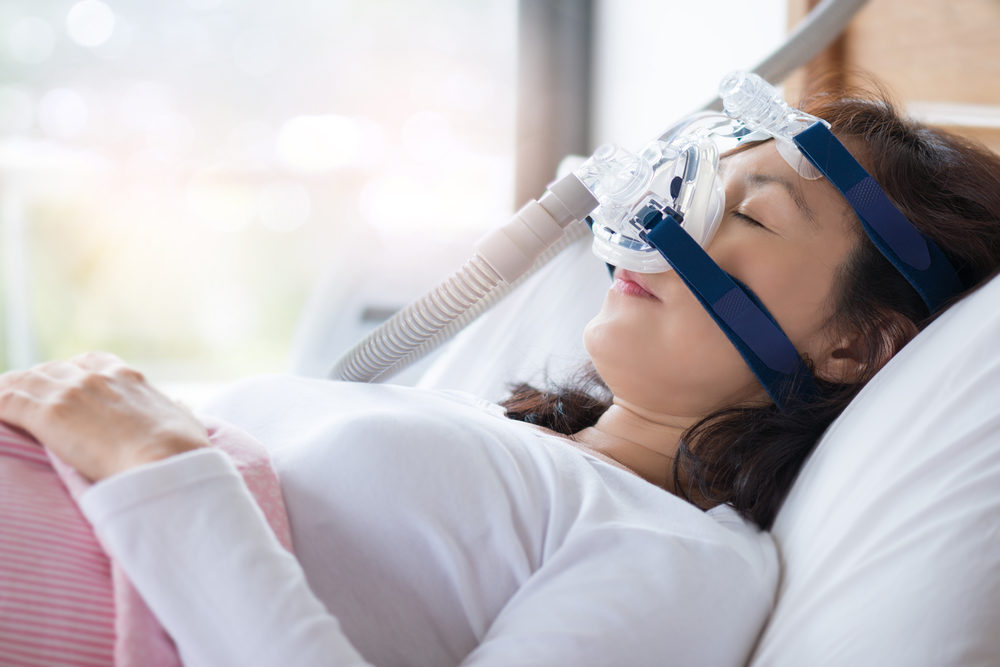
This past April, thousands of people struggling with sleep apnea received disturbing news when Philips Respironics issued a warning about possible defects in some of their respiratory medical devices, especially CPAP (continuous positive air pressure) machines.
The devices use a hose connected to a mask or nosepiece to deliver steady air pressure that regulates breathing while asleep. Most sleep apnea patients rely on them to sleep and prevent serious complications such as stroke, heart attack and even death.
Two months later, on June 14, Philips took the warning further by announcing a sweeping recall. The company determined that the defective CPAP machines were releasing toxic compounds while in use during a breakdown of a polyurethane foam designed to abate the loud operating sound. This degradation was releasing potentially cancerous particles into users’ airways.
The severity of this defect led the U.S. Food and Drug Administration (FDA) to declare the recall a Class I, based on the potential for “serious injury or death.’’
Philips estimated that between three and four million machines would be targeted, with more than 50 percent of those in the U.S. The recall included more than a dozen machines, including CPAP devices, Bi-Level positive airway pressure (Bi-Level PAP) devices and mechanical ventilators.
Over the past six months, patients have scrambled to safely continue sleep apnea treatment while Philips devises an FDA-approved repair plan for existing units that will involve replacing the foam with a safer alternative. But these repairs will take extensive time. The recall has been extended until September 2022 due to a lack of immediately available replacement kits.
CPAP patients’ struggles will likely increase as Philips has not offered “loaner” machines while waiting for fixed units. On top of that, new units cost between $500-$1,000 retail, and Medicare and other insurers will not cover a replacement until the old unit is at least five years old.
Distributors, healthcare providers and especially patients are becoming overwhelmed with the issue, and many patients have chosen to fight by taking legal action against Philips.
CPAP Lawsuits and the Decision for Multidistrict Litigation
Because over 100 federal lawsuits have already been filed against Philips over its faulty machines, a federal judicial panel recently granted the motion to centralize pretrial proceedings into multidistrict litigation (MDL). This means that most individual plaintiffs – including those in New Jersey – will still have their trial, but the pretrial process will be streamlined to save both sides time and money. This is good news for plaintiffs and their families who are struggling to find solutions.
The Judicial Panel of Multidistrict Litigation (JPML), which decides whether to grant MDL, met on Oct. 8 to determine that the cases were similar enough to have “common questions of fact.” The panel consists of seven circuit or district judges.
Included in the JPML’s review were thirty personal injury claims, all determined to be appropriate for MDL due to requiring “…common discovery regarding the development and safety of the recalled devices and the potential harm that can be caused by the alleged defect.’’
Steps to Follow After CPAP Recall
If you have sleep apnea and have suffered due to one of Philips’ recalled machines, you should contact your doctor to have your health condition evaluated and determine your next steps. Your doctor may recommend that you keep or stop using the machine based on your individual factors. You should also register your device on the Philips recall website by entering its serial number.
It is also important to report any symptoms to the FDA’s MedWatch Adverse Event Reporting Program, so the agency knows how many people have been detrimentally affected.
Finally, consider contacting a New Jersey attorney with experience in recalled devices and defective products to discuss possible legal options. If you or someone you know has been affected by the Philips CPAP and BiPAP device recall, the New Jersey lawyers at D’Arcy Johnson Day can help answer any questions. For a free legal consultation, call us at 866-327-2952 or contact us online.

As a partner with D'Arcy Johnson Day, Andrew D'Arcy has been involved in some of the nation’s most high-profile cases and investigations. His practice includes serious automobile accidents, medical malpractice, wrongful death and product defect cases. Andrew has been personally responsible for numerous multi-million dollar settlements and verdicts on behalf of his clients. He has been recognized by his peers as an "AV" rated attorney, the highest possible rating given by Martindale-Hubbell publication. Andrew has been named a "Super Lawyer" by New Jersey Monthly magazine each year consistently since 2013.










Comments for this article are closed.